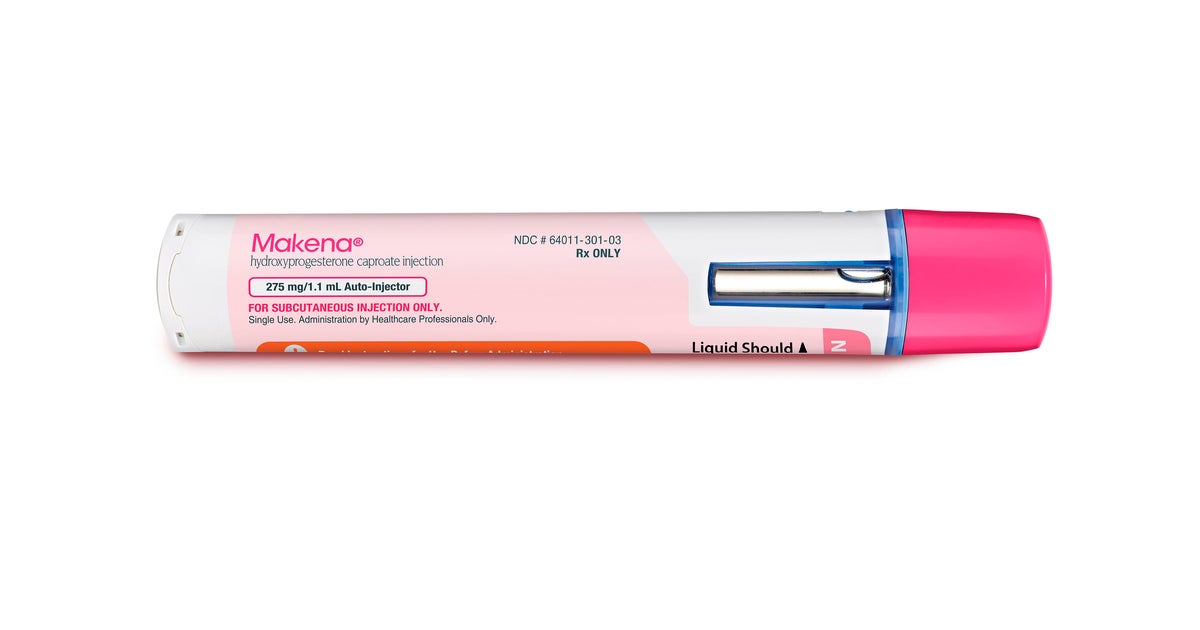
MIAMI — On Thursday, the US Meals and Drug Administration declared its final final decision to withdraw its approval of Makena, a drug accepted additional than a ten years ago to decrease the risk of preterm delivery. The Food and drug administration states that the drug is not productive and that the benefits of using it do not outweigh the chance.
“It is tragic that the scientific investigate and clinical communities have not nonetheless found a remedy shown to be powerful in stopping preterm beginning and improving upon neonatal results — specifically in mild of the actuality that this severe affliction has a disparate influence on communities of coloration, especially Black girls,” Food and drug administration Commissioner Dr. Robert Califf stated in a assertion. “Basically, on the other hand, the touchstone of Fda drug acceptance is a favorable gain-chance evaluation devoid of that favorable evaluation, the drug ought to not have the status of being Food and drug administration-accepted.”
Powerful Thursday, the agency suggests, Makena and its generics are no extended authorised and simply cannot lawfully be distributed in interstate commerce.
Very last month, the maker of the drug said it was moving to withdraw the medication from the industry after an Fda panel reported it is not effective.
“Though we stand by Makena’s favorable hazard-profit profile, such as its efficacy in women at maximum risk of preterm start, we are looking for to voluntarily withdraw the item and function with the Fda to effectuate an orderly wind-down,” Covis Pharma Chief Innovation Officer Dr. Raghav Chari explained in a information release at the time.
In October, the FDA’s Obstetrics, Reproductive and Urologic Prescription drugs Advisory Committee voted that Makena need to not remain on the current market soon after a massive analyze unsuccessful to display that it was productive. It also voted that a postmarket trial did not display any reward to toddlers and that the evidence didn’t exhibit that Makena decreased the threat of preterm start in women who had had a single before.
Covis said that quickly soon after the committee listening to, it outlined a strategy for withdrawal that bundled a wind-down period permitting people to complete the 21-week program of treatment method. Nonetheless, the FDA’s Heart for Drug Evaluation and Exploration turned down the plan.
The Food and drug administration claimed although the approvals of Makena and its generics have been withdrawn, the agency acknowledges that there is a provide of product or service that has presently been dispersed. It mentioned people who have thoughts ought to converse to their wellbeing treatment company.
Thanks for reading CBS NEWS.
Create your free account or log in
for more features.